[ad_1]
The Meals and Drug Administration accepted a request by Eli Lilly on Wednesday to start advertising its tirzepatide remedy, which is branded as Mounjaro for diabetes, beneath a brand new model for weight reduction as nicely.
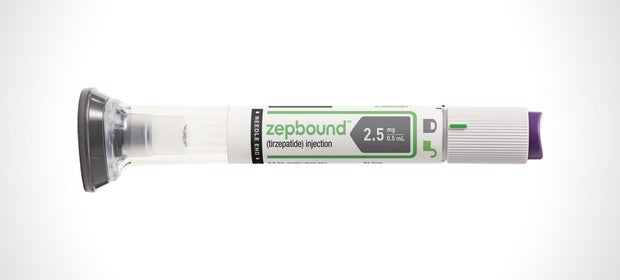
Whereas Mounjaro had already been utilized by some sufferers “off-label” for weight reduction, the brand new FDA approval will permit the drugmaker to start formally promoting and advertising tirzepatide — branded as Zepbound — for weight reduction too.
Zepbound shall be accessible for sufferers within the U.S. by the top of the 12 months, the drugmaker mentioned.
The corporate mentioned Wednesday in a information launch that the remedy, administered with an injection pen, shall be bought at a less expensive listing worth than its semaglutide opponents from Novo Nordisk, that are branded as Wegovy for weight reduction and Ozempic for diabetes.
“New therapy choices convey hope to the many individuals with weight problems who battle with this illness and are in search of higher choices for weight administration,” Joe Nadglowski, CEO of the Weight problems Motion Coalition, mentioned in Eli Lilly’s launch. The group receives funding from Eli Lilly and different pharmaceutical and well being care corporations.
The FDA’s approval of Zepbound was partially primarily based on a trial of adults with out diabetes, which discovered that individuals — who averaged 231 kilos initially of the trial — who got the very best accepted dose misplaced round 18% of their physique weight in comparison with placebo.
Eli Lilly
“In mild of accelerating charges of each weight problems and chubby in america, at present’s approval addresses an unmet medical want,” the FDA’s Dr. John Sharretts, director of the company’s Division of Diabetes, Lipid Problems, and Weight problems, mentioned in a information launch.
Whereas there haven’t been outcomes from giant scientific trials evaluating Novo Nordisk’s and Eli Lilly’s drugs head-to-head, there’s some analysis to recommend Zepboud may outperform Ozempic.
A meta-analysis introduced on the annual assembly of the European Affiliation for the Examine of Diabetes in October concluded tirzepatide was “more practical for weight reduction than semaglutide, with a bigger weight-loss impact at larger doses,” however acknowledged limitations in making an attempt to make a direct comparisons of the 2.
In a report earlier this 12 months evaluating semaglutide and tirzepatide for diabetics, the Institute for Scientific and Financial Overview concluded that tirzepatide confirmed “larger discount” in weight and different key markers, however “had a larger incidence of gastrointestinal negative effects, extreme adversarial occasions, and discontinuation in contrast with semaglutide.”
Zepbound carries the danger of an array of potential negative effects, the FDA says, together with nausea, diarrhea, vomiting, constipation, and hair loss.
Like with different weight reduction medicine on this class, a few of Zepbound’s negative effects might be severe.
Folks with a historical past of extreme gastroparesis, or abdomen paralysis, mustn’t use the drug, the FDA says.
Eli Lilly and Novo Nordisk have each confronted claims that their medicine could cause abdomen paralysis. The FDA just lately moved to acknowledge experiences of ileus, or a blockage within the intestines, on Ozempic’s label.
The company additionally notes that different folks might be at larger danger of extra extreme points from Zepbound, together with sufferers with a historical past of medullary thyroid most cancers, pancreas irritation, or extreme gastrointestinal illness.
It additionally shouldn’t be mixed with different so-called GLP-1 receptor agonist medicine, which embrace its sibling Mounjaro, in addition to Wegovy and Ozempic.
“The security and effectiveness of coadministration of Zepbound with different drugs for weight administration haven’t been established,” the company says.
[ad_2]
Source link