[ad_1]
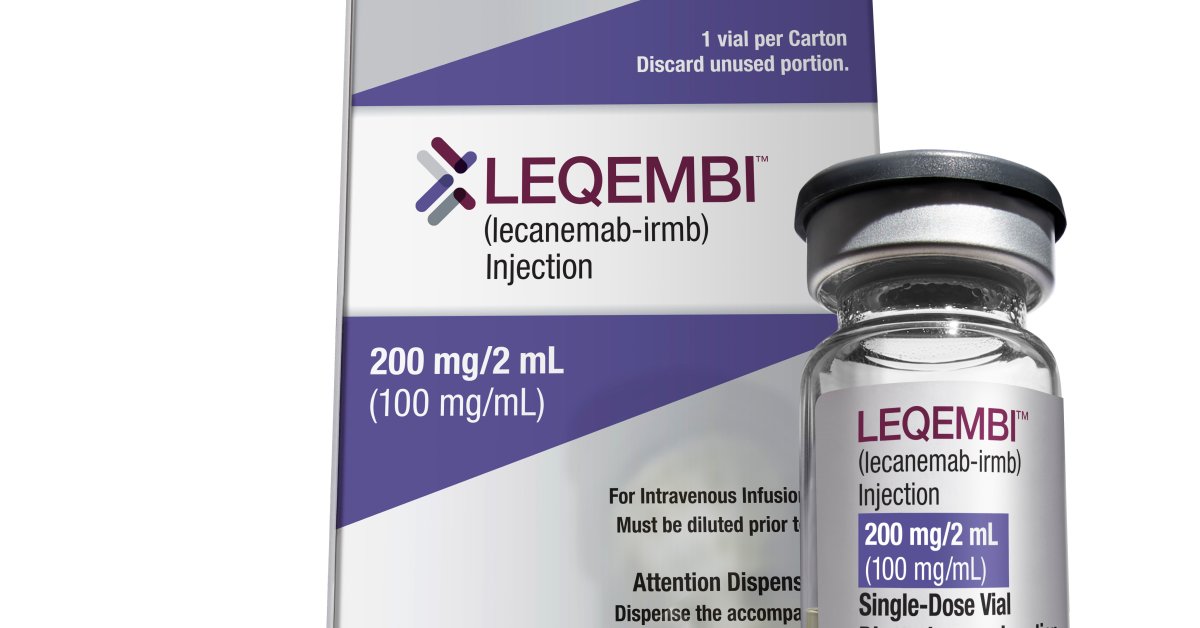
Jay Reinstein, a former assistant metropolis supervisor in Fayetteville, N.C. who was recognized with Alzheimer’s illness in 2018, lastly acquired some excellent news lately. His physician informed him he can be candidate for the newly accredited drug lecanemab (Leqembi). He’s within the early levels of the neurodegenerative situation, which is when the drug seems to be the best. Research present that for folks like Reinstein, twice month-to-month infusions may sluggish cognitive decline by as much as 27%. Maybe extra importantly, lecanemab additionally appears to assist folks proceed their each day actions for an extended time period in comparison with these not taking it. “The brand new drug approval gave me and my household hope,” Reinstein says.
The dangerous information is that at $26,500 a yr, the remedy is financially out of attain for Reinstein—and so many others. Ivan Cheung, the president of Eisai, Inc., which developed lecanemab, speculates that sufferers might should get the month-to-month infusions for at the least two to a few years earlier than they will doubtlessly shift to a much less frequent upkeep dose. Medicare gained’t cowl it; the company has grouped lecanemab into a category of medication that it says requires extra proof to be able to qualify for protection. It’s solely the second medicine accredited by the U.S. Meals and Drug Administration (FDA) to focus on amyloid, the protein that builds up within the brains of Alzheimer’s sufferers, and Medicare has determined that these first-in-class therapies are nonetheless too new to reimburse with out extra proof. Solely sufferers who’re enrolled in a chosen registry that studies on affected person outcomes will likely be reimbursed for his or her remedy.
However these registries haven’t even been arrange, and as soon as they’re, they is probably not broadly accessible to the 6.5 million folks residing with Alzheimer’s, a few of whom are within the early levels and who would possibly profit from the remedy. “I’m indignant, as a result of I really feel we’ve been discriminated towards,” Reinstein says.
The frustration amongst sufferers like Reinstein is rising, and advocacy teams are calling out the Facilities for Medicare and Medicaid Companies (CMS), which oversees Medicare, for including remedy protection restrictions that weren’t put in place for different first-in-class therapies to deal with ailments like HIV or most cancers. Legislators proposed a invoice final November that will forestall CMS from limiting entry to total lessons of accredited medicine with out evaluating the deserves of every individually.
“How will you proceed to boost our hopes after which say, ‘We gained’t pay for it’?” says Jim Taylor, who based the affected person advocacy group Voices of Alzheimer’s together with his spouse Geri (who was recognized with the illness a couple of decade in the past). “It’s continued discrimination towards folks with the illness.”
No time to waste
The truth that lecanemab appears to be only throughout the earliest levels of the illness makes the coverage much more of a gut-punch for sufferers and households. “I’m younger sufficient and energetic sufficient that this may be the fitting time for me to begin this drug,” says Reinstein. “So I’m feeling a way of urgency; I need the development of my illness to doubtlessly be slowed down.” Every day, the Alzheimer’s Affiliation estimates about 2,000 folks transfer from the delicate stage of illness to the average part, at which level the drug turns into far much less efficient. The weeks or months it should take to arrange the registries required by CMS imply that individuals who slip into extra average and superior illness will change into ineligible for remedy.
“How will you defend that?” asks Taylor, who says that his group, Voices of Alzheimer’s, is ready to persuade CMS to rethink its coverage by any means potential. They’re planning to publish a white paper on what it says is a legacy of discrimination towards folks with Alzheimer’s and dementia—and planning extra disruptive ways as properly. “We would do a die-in in entrance of the Division of Well being and Human Companies constructing,” says Taylor, referring to a protest tactic during which activists lie on the bottom mimicking corpses, “or a sit-in. We wish to do every part we are able to to name consideration to the untenable place CMS holds.”
A historical past of heartbreak
Alzheimer’s sufferers have been on a curler coaster of hope and frustration over the previous a number of years. After a long time of getting solely drugs to deal with Alzheimer’s signs, however not its root causes, the FDA accredited the primary disease-modifying remedy, aducanumab, in 2021. However the knowledge supporting the approval, and the drug’s effectiveness, had been controversial; one main research confirmed advantages to sufferers, whereas one other didn’t. Based mostly on that uncertainty, CMS positioned aducanumab in a class referred to as Protection with Proof Growth (CED), a designation that solely offers reimbursement if sufferers are enrolled in both a medical trial to assemble extra info on the drug or a registry so the corporate and consultants can proceed to observe for unwanted effects and the way properly the sufferers do.
Due to how controversial the FDA’s approval was, CMS additionally determined to position any related future medicine—monoclonal antibodies designed to seek out and bind to amyloid—beneath the identical restrictions. That included lecanemab, which was accredited in Jan. beneath an accelerated approval pathway. Even when lecanemab acquired conventional approval from the FDA, which is anticipated in some unspecified time in the future this yr, the requirement for reimbursement will stay the identical: sufferers should be a part of a registry to be able to get lined.
For Alzheimer’s sufferers, that dampens any optimism that the approval of the medicine raised. “The FDA has accredited different medicine for most cancers, AIDS and different issues, and CMS has accredited them, so why is it dragging its ft on an Alzheimer’s drug?” says Reinstein, who serves on the board of administrators for Voices of Alzheimer’s.
Taylor believes he is aware of why: “They’re attempting to save cash,” he says. “They don’t have the funds to pay for a drug for which tens of millions will likely be eligible. Even when they aren’t supposed to think about value, that’s the first motivator to proceed what we really feel is a historic discrimination towards folks with Alzheimer’s and dementia.”
Susan Peschin, president and CEO of the Alliance for Ageing Analysis, a nonprofit group targeted on supporting efforts towards wholesome getting older, agrees. “[CMS] is attempting to manage the movement of those companies for Medicare to save cash, beneath the guise of proof assortment,” she says.
In a written response to TIME, CMS stated that the company is “dedicated to creating efficient therapies accessible to folks with Medicare,” noting that lecanemab is roofed if sufferers meet the registry requirement for amassing extra info on the drug. A spokesperson stated CMS “continues to actively have interaction with all stakeholders and is reviewing knowledge that will reply these Protection with Proof Growth (CED) questions” and “encourages any stakeholder to ship related knowledge to help in a reconsideration of the present protection.”
Maria Carrillo, chief science officer of the Alzheimer’s Affiliation, says these necessities solely delay getting the remedy to sufferers who want them essentially the most. Whereas the Affiliation at present has a community of medical doctors and amenities, often known as ALZ-NET, that collects info on sufferers handled with Alzheimer’s therapies and might be used a framework for gathering the information on lecanemab, Carrillo says establishing the required registries to satisfy CMS’s standards would take months, if not years. “We actually can’t afford to attend for the restricted alternative [to access] that CEDs create,” she says. “We anticipate, with the present accelerated approval, and with full approval, that there will likely be protection [of lecanemab] instantly.”
With every day that passes, Reinstein feels his hope—and cognitive skills—dissipating. He not drives, and his studying retention and short-term reminiscence are beginning to slip away. Giving up his driver’s license devastated him. “Now I’ve misplaced my independence; what’s subsequent?” he says. “All I take into consideration are my grandbabies, and spending time with them and my three youngsters. The brand new drug approval gave me and my household hope. Now it’s time to only get me the rattling drug.”
Extra Should-Reads From TIME
[ad_2]
Source link



:max_bytes(150000):strip_icc()/Health-GettyImages-StrongGlutes-d834d403c3824ecc947fd2e1272beedc.jpg)






















